The 'planetary model' of the atom, where electrons are visualized as tiny particles orbiting the nucleus, is sufficient for a basic understanding of the theory of a laser, so I haven't used a model any more complicated than that one. Also, this is the model most high school students are familiar with. As background, I introduced or reviewed the idea of energy levels: how an electron can occupy different orbits, each of which has a different energy associated with it; and explained that only certain specific values of the energy are possible, which depend on the particular atom. The analogy of rocks on a staircase, as opposed to a ramp, was helpful to explain this concept. I also introduced, or reviewed, the idea of a photon as 'a piece of light' or 'a particle of light' and how the colour of a photon is determined only by its energy.
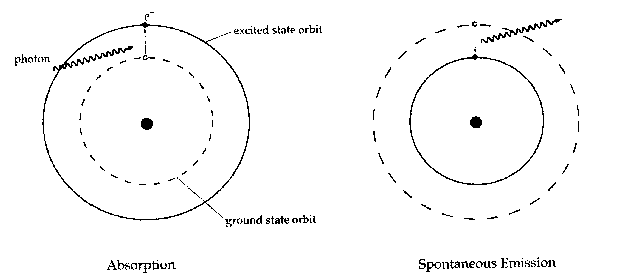
A given electron in an atom has an orbit of lowest energy that it can occupy, called the 'ground state.' If it is in an orbit with a higher energy, it (or the atom containing it) is said to be in an 'excited state.' As it is a general rule that systems prefer to be in a state of lowest energy, the excited state is unstable: if an electron is in an excited state, it tend to stay there for only a short time before jumping back down again, releasing its excess energy in the form of a photon. Since the energy states of a certain atom are well-defined and the same for all atoms of that sort, only photons of certain distinct energies are emitted by any particular substance, as is shown by spectroscopic emission lines.
There are three types of interaction an atom and a photon can have: absorption, spontaneous emission, and stimulated emission. Absorption occurs when a photon of just the right energy for a particular transition encounters an atom in the ground state, and causes the atom (or rather, one of the atom's electrons) to jump into an excited state. Spontaneous emission is when an excited atom spontaneously returns to the ground state, emitting a photon in the process.

However, if a photon of just the right energy hits an atom whose electron is in the excited state, it can induce the electron to jump down to the lower state, emitting another photon, which will be in phase with the incoming photon and travelling in the same direction. This is called stimulated emission.
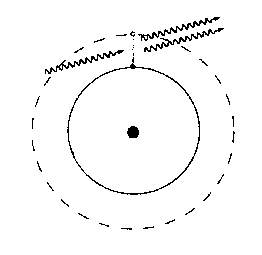
Under normal conditions, for any given transition there are many more atoms in the ground state than in the excited state. In this case, if an excited atom emits a photon by spontaneous emission, the photon is likely to encounter another atom in the ground state and be absorbed. Suppose, however, that a substance can be set up so that, for some particular transition, there are many more atoms in the excited state than in the ground state. This condition is called a population inversion. When one of the excited atoms spontaneously emits a photon, the photon is likely to hit another excited atom, stimulating the emission of another photon. These two in turn will probably hit excited atoms and stimulate more emissions, which will stimulate more emissions, and so on. In this sense, the medium acts as a light amplifier: the original one photon has been amplified to a large number of photons, all in phase and travelling in the same direction.
Now suppose that such a substance is set up in the shape of a rod, with mirrors at either end. Various excited atoms will emit photons, which will travel through the substance for awhile, perhaps stimulating more emissions, and then exit the material. However, if one of the spontaneous emissions happens to be along the axis of the rod, the photons it stimulates will reflect off the mirror at one end of the rod and travel back through it, stimulating more emissions and being amplified further. They will bounce off the back mirror as well, and (as long as they are parallel to the axis) will continue being reflected back and forth, and being amplified as they go. Now if one of the mirrors is made to be partially transmitting (often as little as 1%), then part of the light will escape out that end of the rod. This escaped light is what forms the laser beam.
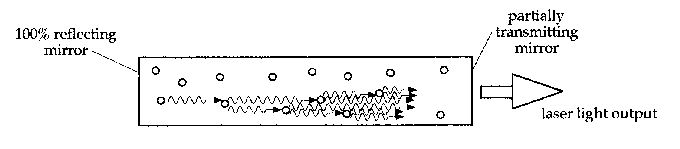
The light thus produced has several important characteristics. Firstly, only photons travelling extremely close to parallel with the axis of the rod will be amplified enough to escape through the mirror. Hence the laser light will only exist in a narrow beam. Secondly, as all the photons are coming from the same transition, they have the same energy and hence the same colour. So the laser light will be monochromatic. Thirdly, since stimulated emission produces another photon in phase with the incoming one, all the photons in the laser beam will be in phase, and so the laser light will be coherent. These three properties are what distinguishes laser light from light produced by other sources.
One of the main difficulties in making a laser is obtaining the necessary population inversion. First, the laser has to be made of material capable of sustaining a population inversion long enough that laser action can occur. Second, there has to be some method of pushing large numbers of the atoms in the material into the excited state. There are many different methods used to bring this about, depending on the laser material. The first laser ever built used ruby crystal as its laser material, and used a pulse of light from a flash tube to excite the chromium atoms in the ruby. In a gas laser, such as a helium-neon laser, an electric current is used to excite the atoms of a gas, the same way as in a conventional neon light, and the population inversion comes about because of the way the gasses interact. In a semiconductor diode laser, such as the laser pointer, the light is produced when electrons in one semiconductor fall into lower energy states in another semiconductor. The process is the same as that which occurs in an LED, and indeed, at low currents, a laser diode produces incoherent light like an LED. A large enough current passing through the semiconductor produces a population inversion and gives laser operation.