Latent Heat of Fusion
At the end of the first demonstration, we have introduced another important fundamental principle of physics: Heat.
Heat is energy transferred from one object to another because of temperature difference.
Strictly speaking, the word heat refers only to energy in transit between systems. The easiest way to quantify this transition is by measuring the temperature difference of the systems. The SI unit of temperature is Kelvin, where it is defined by setting the triple point at exactly 273.16 K. One Celsius degree represents the same temperature difference as one Kelvin (K), but the zero of the Celsius scale occurs at 273.15 K. The Celsius scale was chosen so the melting point of ice at standard atmospheric pressure is at exactly 0oC.
Experimentally, the heat(DQ) transferred to an object and the resulting change in the object's temperature(DT) are directly proportional,
DQ=CDT
where C is the heat capacity of the object. The SI units of heat transfer and heat capacity are joule and joule per Kelvin respectively. Since we want to characterize different substances in terms of specific heat c (joule per kilogram per Kelvin), or heat capacity per unit mass, the above equation can then be written as:
DQ=mcDT
Now, let's apply the above concepts to one of the most important atmospheric substances--Water. The main reason for this is that water is the substance that commonly exists in all three phases at once, where the speed of individual water molecules determines what phase each molecules exists in. The three different phases are water vapor, liquid water and ice.
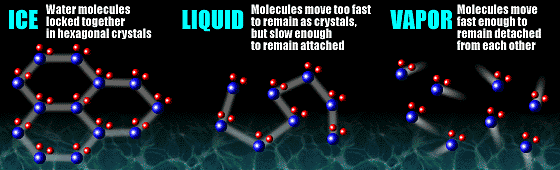
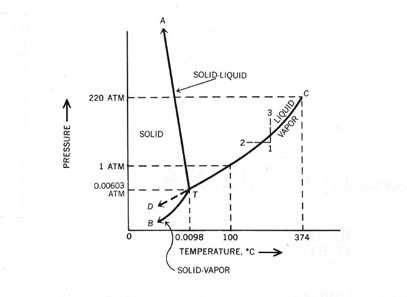
As seen in the graphic above, the slowest moving molecules exists as ice, where the water molecules are locked into a hexagonal crystal and do not move freely. Liquid water molecules are moving fast enough to break free from the crystal structure, but they are still attached to each other. This allows liquid water to assume the shape of its container, for example. The water molecules in the vapor phase are moving fast enough so that they bounce off of each other and do not become attached. The following schematic diagram shows the phase changes of water under different pressure and temperature. Notice the triple point marked on the diagram below showing all three phases of water can coexist (click the following diagram to challenge yourself with some problems related to phase change).
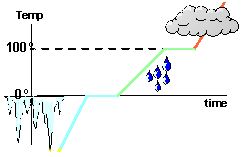
When heat is added to a solid, the kinetic energy of the molecules in the solid increases and the increased motion of the molecules is reflected by a rise in the temperature of the solid. When the melting point of the substance is reached, however, the temperature remains constant as the change of phase occurs. Let's start with a solid piece of a given substance--for example, ice. We start adding heat to the ice. As we do so, the ice warm up. The more heat we add, the more temperature increases. This process does not continue for long, however. If we continue adding energy to the ice, we will find that we will reach a certain temperature at which the addition of more energy does not cause the temperature to increase. Instead, examination of the ice shows that the ice is starting to melt--that is, it is starting to undergo a phase change (as the flat line of the following cartoon).
The term 'latent heat' or 'heat of transformation' refers to the heat change associated with a change of state of phase. It is the heat given up or absorbed by a unit mass of substance as it changes from a solid to liquid, from a liquid to a gas, or the reverse of either of these changes. It is called latent because it is not associated with a change of temperature. For example, the latent heat of fusion, Lf, is given by
Lf=DQ/m
The objective of this demonstration is to show how to determine the latent heat of fusion of ice by melting a known mass of ice in warm water. The materials that needed for this demonstration are as follow:
- A thermometer
- Four different size of beakers (600ml, 400ml, 250ml and 150ml)
- A piece of glass or plastic with an insert that fits the thermometer
- Some ice
- Warm tap water
The procedures are as follow:
The amount of energy lost by the warm water is easily calculated with the formula:
- Stack four different size beakers together (the air gaps between the beakers act as insulation). Fill the center beaker with a known mass of warm water. Record this mass and temperature. To view the set up click here.
- Place two or three pieces of ice into the center beaker and stir until the ice melts.
- Record the final temperature of the water and the final mass of all water in the beaker.
- Calculate the mass of water added by the ice.
Energy change(DQ) = mass*(Tfinal-Tinitial)*c
where c is the specific heat of water, 4184 J/kg/K. Remember that this energy change is negative because the water loses energy. After the ice melts it is liquid water with a temperature of 0oC (273.15 K). This water gains energy until it reaches the equilibrium temperature. Thus, the amount of energy gained by the ice (water after it melts) is slightly more complicated to calculate.Energy change(DQ) = mass*(Tfinal-Tinitial)*(4184 J/kg/K) + mass*Lf
where Lf is the heat of fusion of ice. This energy change is positive because the ice/water gains energy. Using these two equations, solve for Lf and compare the obtained value to the known value.
Mini conclusion:
At the end of this demonstration, students should be able to tell what is going on during the process of phase change and calculate the heat of fusion of ice. They should understand some common thermodynamics terms like temperature, heat, specific heat capacity and the heat of fusion.