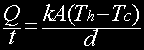| The Theories Behind Heat
Transfer
Definition Heat is a form of energy that is transferred by a difference in temperature. However, some may argue that definition will not hold for radiative cooling. We will come back to cover this issue later. There are 3 types of heat transfer: convection, conduction and radiation. We will explore each of these mechanisms of heat transfer and see how they can contribute to computer cooling. Conduction Conduction is the transfer of heat through a substance by molecular action or from one substance by being in contact with another. When an object is hot, the molecules inside have more kinetic energy. The molecules, having high kinetic energy bounces against its less energetic neighboring molecules transferring the energy. Eventually, the kinetic energy is spread evenly throughout the whole object by molecular collision. We can infer a few things from here: when an object is equal-temperature everywhere, convection will not occur, since all the molecules are vibrating with the same kinetic energy, bouncing off each other will not cause any change in energy given that each collision is inelastic. The equation that governs convection is:
This equation is fairly easy to understand. The amount of heat transferred is linearly proportional to the ability of the material to conduct heat (k). The bigger the area in contact the faster heat will transfer. The bigger the different is between the cold and the hot end, the faster heat will transfer. On the other hand, the longer the distance you have to transfer heat through, the slower the heat transfer will be. In computer cooling, we can vary the material we use for the case to increase the heat dissipation from the heat sources. From the table we can see that the conventional material, steel is not as good as aluminum in terms of thermal conductivity. That's why newer/better computer cases are using aluminum cases instead of steel.
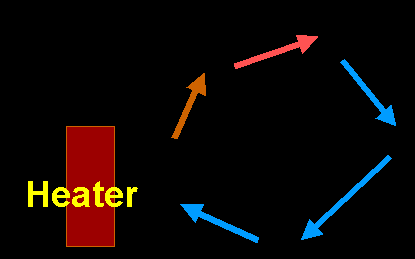
Ice has low thermal conductivity, that's why it can be used to build Igloos, although it's not as good as wood. Air is an excellent thermal insulator (as oppose to conductor). You can stay warm by just wearing a layer of air. However, this is only possible if you lock the air in that layer (down jacket), otherwise the air will start to move and convection will occur and you'll begin to lose heat. Convection Convection is the transfer of heat by mass motion of a fluid such as air or water when the heated fluid is caused to move away from the source of heat, carrying energy with it. The movement is caused by the change in density. Take air for an example, as the air around an object absorbs heat from the object through molecular collision on the surface, it heats up. As air heats up its volume increases, while the mass stays the same. The results in a decrease in density. In the same medium, lighter air then moves up carrying the heat away from the object. The cooler air then fills into where the hot air were and repeats the cycle. This cooling method is best illustrated with this diagram:
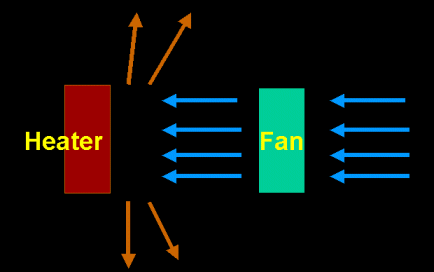
The above method can be categorized as "nature convection" whereas there is the "forced convection." In forced convection, the currents are generated by means of pumping or blowing to speed up the convection process.
Convection is not only limited to air. Media such as water or mercury can be used to create convection as well. In computer cooling, water is more preferable than air because it has a higher specific thermal capacity. Specific thermal capacity indicates how much heat energy a kilogram of material can take when it's heated up by 1 Kelvin. In another word, in order to heat up a kg of copper by 1 Kelvin you will need 386 Joules of heat
Now we have seen two properties of a material. Thermal conductivity k and specific thermal capacity. Thermal conductivity measures how fast a material can transfer heat and specific thermal capacity measures how hot a material get when a certain amount of heat is transferred to it. In computer cooling, we want material to be good at heat transferring, but we don't want it to stack up a lot of heat. Aluminum has a good high k value, but also has a high specific thermal capacity. This means that the although aluminum will transfer heat efficiently, it will tend to "stack up" the heat inside. Copper, on the other hand, has a higher k value than aluminum but a lower specific thermal capacity, meaning that it will get much hotter than aluminum given the same amount of heat. As we've seen before, conduction depends on the different in temperature. So copper getting hot easily actually helps the conduction to transfer heat faster. Copper has roughly the same raw material price as Aluminum, yet is better for cooling, why then isn't copper used for computer cases? Ironically, the difficulty originates from specific thermal capacity. As coppers are being machined into shape the friction produces a lot of heat. As we've learned, copper gets hot fast with heat, making it difficult to work with. Adding to that coppers are softer than aluminum and denser. However, for small dimension components such as CPU heatsink, coppers are slowly being introduced. With better manufacturing techniques copper computer cooling components will be more common. Getting back to Convection, for the convection medium, because our aim is to move heat away from the heat source using convection current circulation, it is more ideal if the medium has a high k value. With the high k value the convection current can more efficiently move the heat away from the source. From another point of view, there's only a limited amount of space inside a computer enclosure, we don't want the medium to heat up too quickly and therefore we need a high specific thermal conductivity. In sum, water-cooling is better than air cooling. However it is mostly just because of the higher k value of water compared to air and less due to the specific heat capacity. It is said that liquid mercury can be used as a forced convection medium since its k value (8.3 J/msK)is higher than of air. Yet the pumping power required and the toxicity of mercury might raise some concerns. In conventional computers, heat sources are air-cooled. Understanding convection, we should put the hotter components on at the top of the case so that the heated convection current doesn't transfer heat to other components. It is also essential that the heated convection current can be removed from the cooling system (via fan or exhaust) to increase convection efficiency. Radiative Cooling Radiation is the heat transfer by the emission of electromagnetic waves which carry energy away from the emitting object. The definition we had for heat transfer was: Heat is a form of energy that is transferred by a difference in temperature. It can be argued that radiation occurs without any temperature difference. A cold object can radiate energy out just as hot objects. However, if we strictly look at two objects, one hot and one cold. Both objects will radiate but the net energy transferred is in the direction from hot to cold. Radiation is governed by the equation:
We can already see the similarities
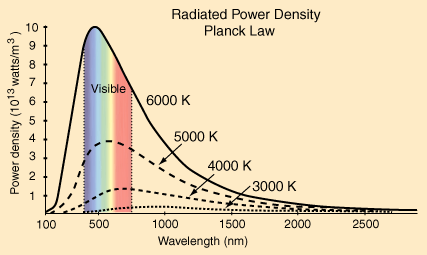
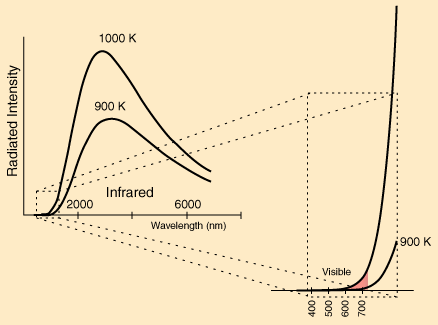
between this equation and the equation for conduction. Here The energy radiated by a heated object are in the forms of electromagnetic waves (lights). However, the human eyes can only detect a limited range of wavelengths. At lower temperature, the light emitted by the heat source are invisible to us. Let's look at several wavelength distribution of light at different temperature.
At extremely high temperature such as the sun at 6000K, the emitted radiation is clearly perceived by the human eyes.
However, at low temperature, the radiation at best is a pale pink. This can be seen from the forging of metals.
Since at low temperature (below 500K) radiation does not transfer a lot of energy. We can safely rule out the radiative cooling factor of computer cooling, since we're only operating at 30~70 Celcius (300K~340K) |