Compression
There are two forms of compression that can occur adiabatic compression and isothermic compression.
Adiabatic Compression
No heat escapes the system or is introduced to the system while the compression is occurring. This is very key no energy enters or leaves the system as HEAT while it is being compressed or expanded, energy may still enter of leave a system via the work preformed on/by the system. Heat is a rather slow form of energy transfer, so in order to have no heat enter or escape from a system undergoing an adiabatic process the expansion/compression is done rather quickly. From the first law of thermodynamics
U = Q + W
During an adiabatic process no energy is lost as heat which implies
Q = 0
So,
U = W
or the internal energy of the system is altered only by the work done on the system.
Isothermic Compression
Isothermic (from the Greek meaning “same temperature”) compression doesn't allow the temperature in a system to change. From the ideal gas law
PV = NkT
After a little rearranging
T = (PV)/(Nk)
if the number of molecules are held constant and a proportionality sign is inserted rather than a equality
T ά PV
As the volume decrease the pressure increases, this is not a balance however the pressure increases fast then the volume decrease. This tells us that as the volume decrease (and such the pressure increases), the temperature also increase and visa versa for expansion.
Recall that the temperature in an isothermic process doesn't change but as we just derived with the ideal gas law, during compression the temperature increase. What this means is that as the system is compressed (remember also that compression is doing work on the system), heat must escape the system to keep it at a constant temperature. So in isothermic compression temperature is constant but heat does escape or enter the system. The amount of heat entering the system is equal to the amount of energy added to the system by doing the compression work.
Q = -W
if this is applied to our formula from the first law of thermodynamics (the conservation of energy)
ΔU = Q + W
ΔU = -W + W = 0
So during isothermic compression U is constant.
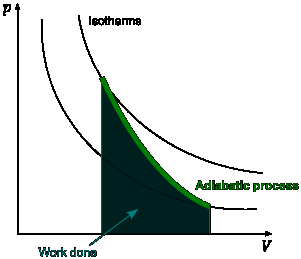
PV Diagrams
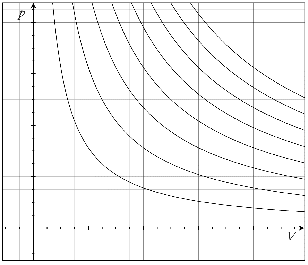
PV diagrams are graphs of pressure vs volume respectively. Isotherms ( Graphs of isothermic processes) have the constraint that throughout each line pressure multiplies by volume has to be equal to a constant.
PV = Constant
The graph below shows several isotherms each holding the constraint.

Adiabats connect isotherms together allowing one to switch between two different isotherms. The constraints on adiabats are more complicated then the constraints on isotherms and are beyond the scope here. Below is an example of an adiabate.