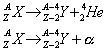
Alpha
Alpha particles are the most massive of the three ionizing radioactive particles. It is a Helium nucleus. It consists of two protons and two neutrons. Unstable elements can be thought of as having too much positive charge for the nucleus to hold together. This means that the nucleus is unstable. To become stable, alpha particles are emitted. This can be represented by the following equation
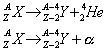
where A is the number of protons and neutrons, and Z the number of protons, an element X has.

While alpha particles have a lot of mass as compared to the other two particles, alpha particles react readily, and have very little penetration power. Thus when we are exposed to alpha particles, most of the particles react with our outer dead skin layer. During the late 1800's miners were subject to very high rates of lung cancer. Much of this cancer was caused by alpha particles. As was mentioned, alpha particles have very little penetration power. External sources of alpha particles are very unlikely to cause lung cancer, even in large amounts for this very reason. The deep mines were, however, subject to large pooling of radon gas. This gas is part of the chain of Uranium decay. Because it is a gas, it can be inhaled into the lungs. There, because of the very fine capillary size, the alpha particles emitted are now able to increase the probability of a person developing cancerous cells. This is the way that the miners got lung cancer. Ventilation into mine shafts has been able to greatly overcome this problem. Radon gas does, however, still tends to accumulate in large cement basements. The effects of this type of radiation exposure was popularized by the media in the 1980's.Radon gas detectection
Gamma Radiation
Gamma radiation is a very high energy photon. Everyday light that we see can be thought of in two ways, as a particle or as a wave. When we think of it as a particle, the particles are called photons. Photons also serve to carry the electrostatic force.
Often when a nucleus undergoes alpha decay, it is left in an excited state. As the nucleus returns to its ground state, a high energy photon is emitted. This is called gamma radiation. It can be represented by the equation
![]()
where X*, represents an element X whose nucleus is in an excited state.
Of the tree types of radiation, gamma particles have the most penetrating power. These particles can easily pass through a person, or a thin sheet of lead. They are stopped by thick lead shielding.
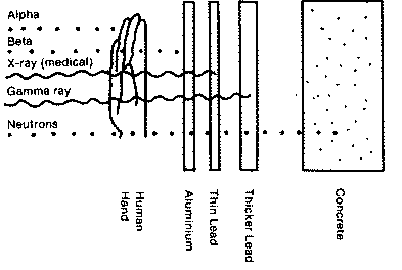
Beta radiation is composed either of high energy electrons, or the anti-matter counter part, positrons. Positrons are exactly the same as electrons, except that they have one positive charge rather than a negative charge. Free electrons of much, much lower energy, and thus danger, are produced by TV's and computer screens. Inside a TV, electrons are fired out towards the screen. There they react to produce the colors we see. Standing in front on a TV, we are directly in the line of fire of these electrons. Some new computer monitors advertise "low radiation" screens. In addition after market screen covers are advertised as not only protecting your eyes, but shielding
The high energy and hence danger of beta particles is a result of the reactions that cause their emission. Usually elements that have too many neutrons to be stable emit beta rays. Ordinary beta decay occurs when a neutron is lost and a proton is gained. This results in the emission of a high energy electron, and another particle called the anti-neutrino. The anti-neutrino is just the anti-matter counterpart of the neutrino, one of a long list of elementary fundamental particles. The reaction is shown by
![]()
Another method of beta decay is proton decay. This is when a proton becomes a neutron and, a positron and a neutrino are emitted
![]()
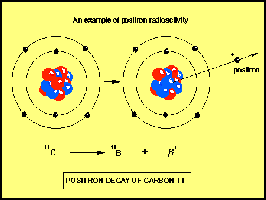
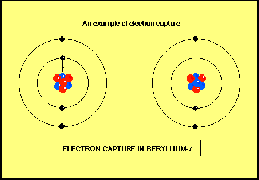
The third way in which beta decay con occur is by electron capture. There is some chance that the innermost electron in an atom will be found in the nucleus. When this happens, a proton changes into a neutron, and a neutrino is ejected.